![PDF] Good manufacturing practice and clinical-grade human embryonic stem cell lines. | Semantic Scholar PDF] Good manufacturing practice and clinical-grade human embryonic stem cell lines. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a9253f34edd2e270047431cf7e75f4f38c4afdc1/4-Figure1-1.png)
PDF] Good manufacturing practice and clinical-grade human embryonic stem cell lines. | Semantic Scholar

Frontiers | Advanced Therapy Medicinal Products' Translation in Europe: A Developers' Perspective | Medicine

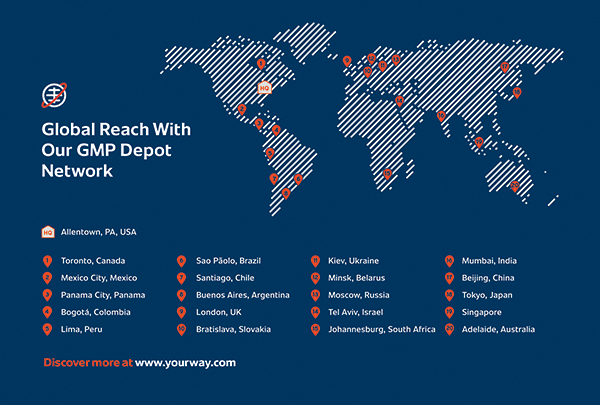
Good Manufacturing Practice in China: Equipment Strategy and Quality Management to Compete with the West - BioProcess InternationalBioProcess International

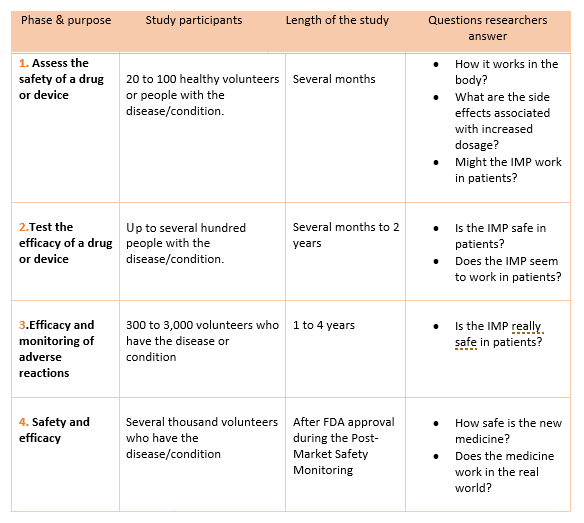
Regulatory Compliance in Pharmaceutical Development: GLP & GMP Jeffrey G. Sarver, Ph.D. MBC 3100 March 8, questions to: - ppt video online download

Quality Management Systems - Woodley BioReg Regulatory Affairs, Compliance and Conformance for pharmaceutical, biopharmaceutical, healthcare, API and Medical device manufacturers
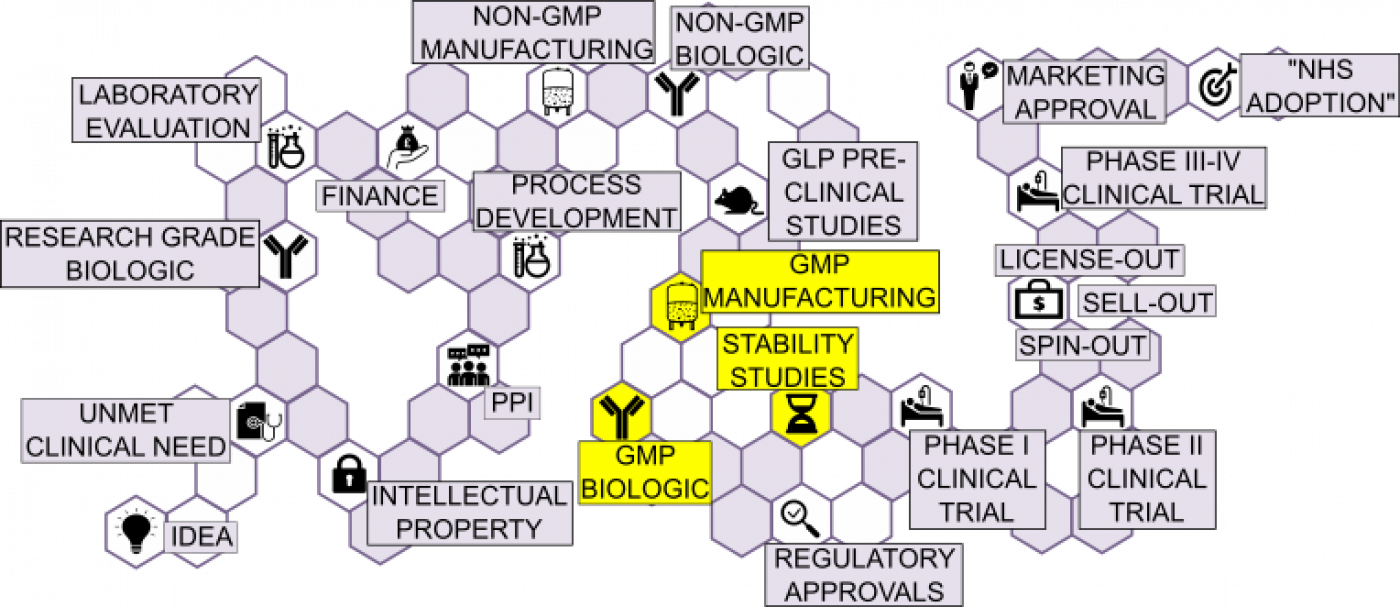
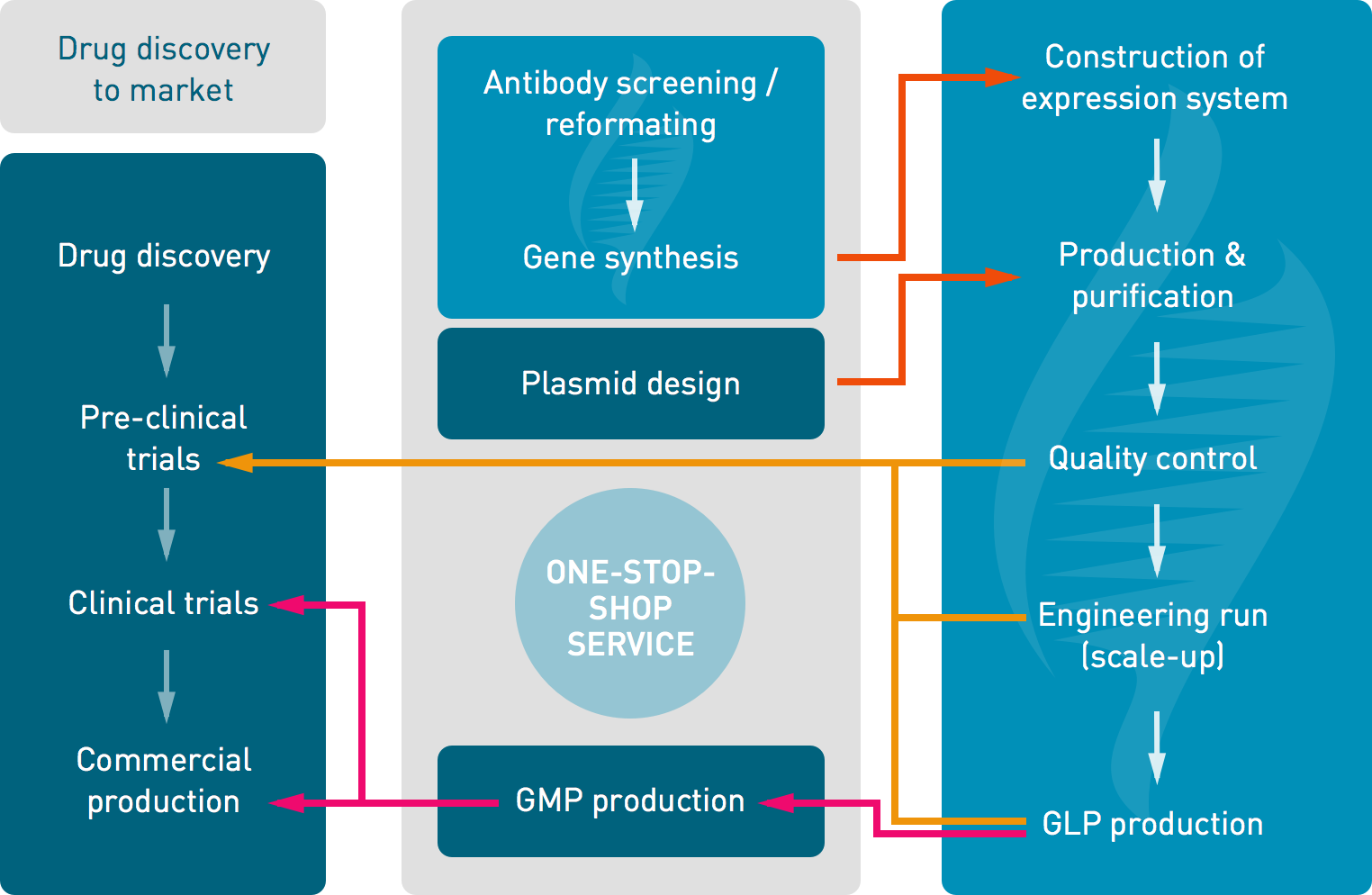
Manufacturing a Clinical Trial Product (Biologics) | UCL Therapeutic Innovation Networks - UCL – University College London







![The Quality System in the pharmaceutical context [Grandi Strumentazioni e Core Facilities (FAST)] The Quality System in the pharmaceutical context [Grandi Strumentazioni e Core Facilities (FAST)]](https://corefacilities.iss.it/dw/lib/exe/fetch.php?media=en:aree:fabiocell:sq.png)